SPRAVATO® offers a flexible and innovative approach to treatment in treatment-resistant depression (TRD)
SPRAVATO® dosing schedule
A Phase III, randomised, double-blind, active-controlled, multicentre trial designed to assess and compare the ability of fixed-dose SPRAVATO® + newly-initiated oral antidepressant (AD) to improve depressive symptoms vs. an active comparator of placebo nasal spray + newly-initiated oral AD in adult patients aged 18 to 64.
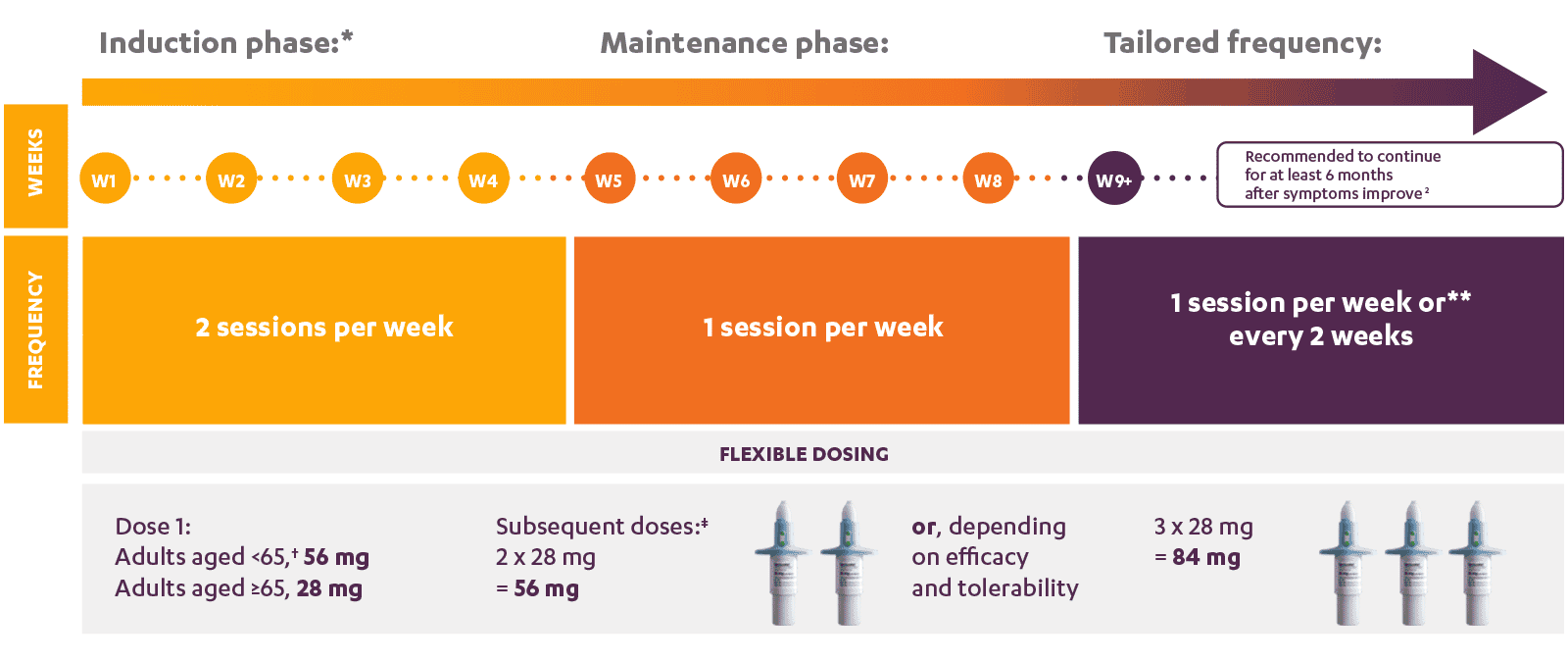
SPRAVATO®, in combination with a SSRI or SNRI, is indicated for adults with treatment-resistant Major Depressive Disorder, who have not responded to at least two different treatments with antidepressants in the current moderate to severe depressive episode.[^2] SPRAVATO® must be co-administered with a SSRI or SNRI. Adults aged ≥65 may receive subsequent doses at 28 mg.
SPRAVATO® does not require dose adjustment to account for:

Weight

Gender

Renal impairment§

Mild hepatic impairment||

Concomitant medications¶
Certain medications should be used with caution in combination with SPRAVATO®¶
SPRAVATO® dosing frequency can be adjusted to maintain clinical benefit
For patients achieving remission, dosing frequency can be reduced and benefit maintained#,
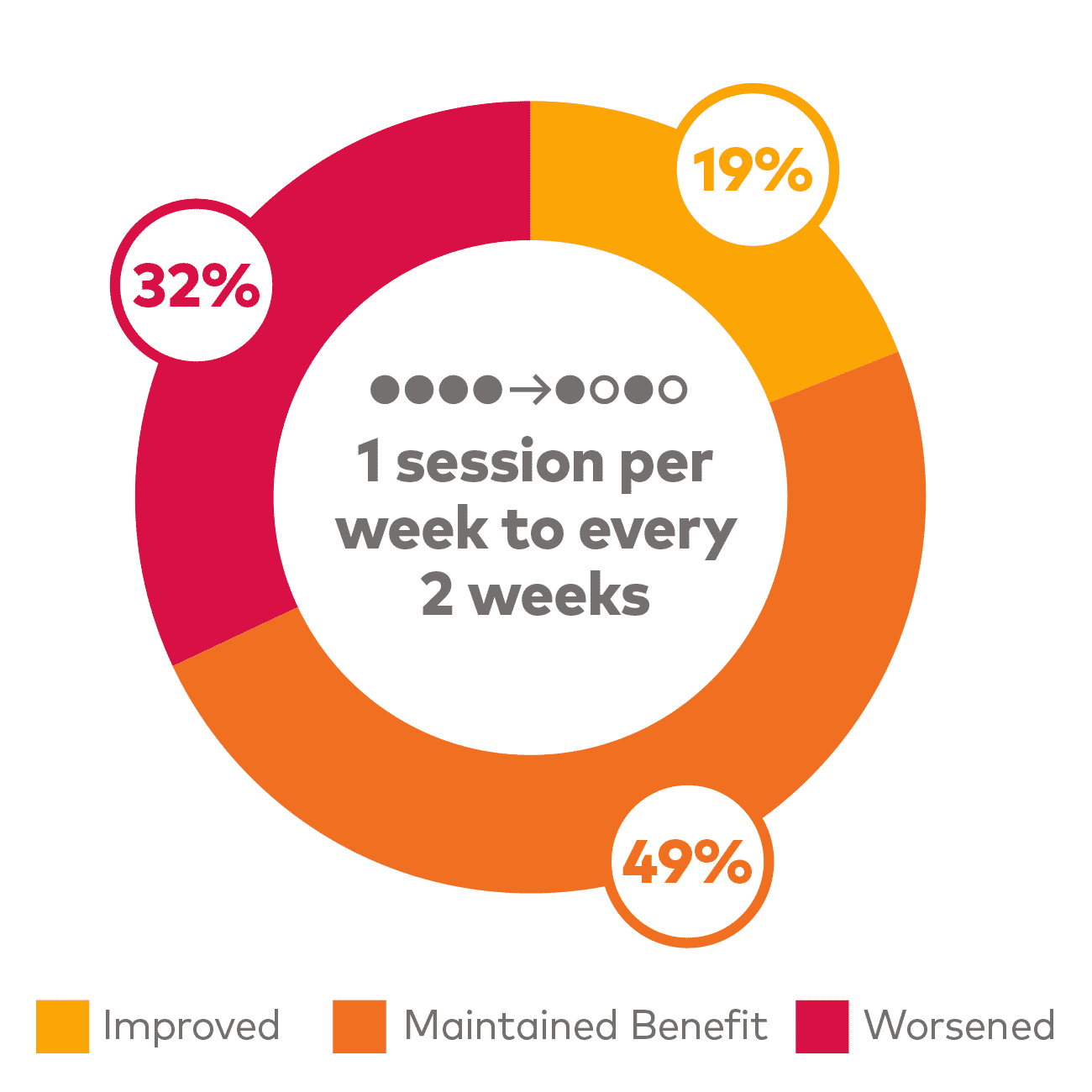
If patients lose their remission status, increasing the dose can recover positive outcomes#
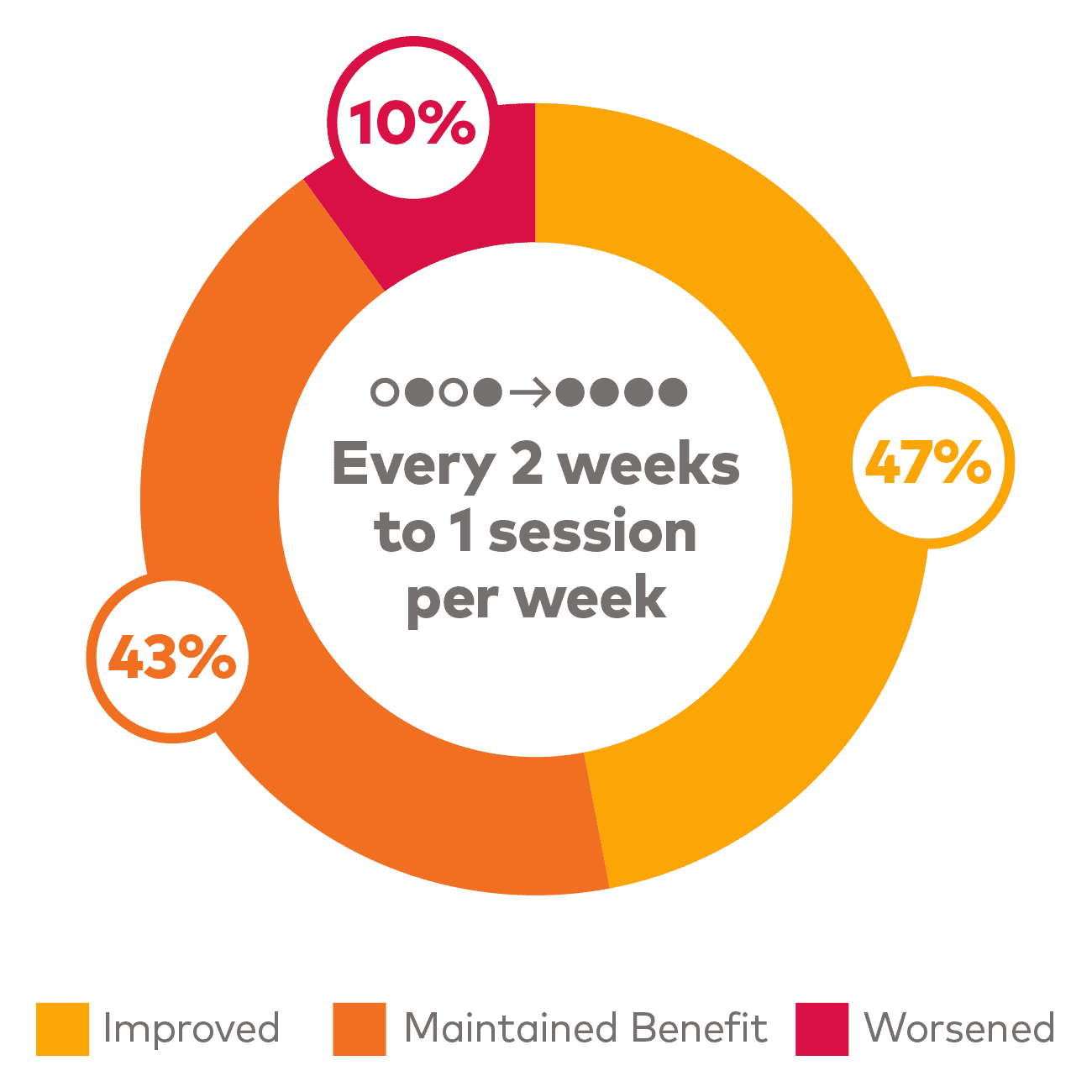
Adjust SPRAVATO® dosing frequency to increase the likelihood of positive outcomes
Adapted from Nijs M et al. 2020.
SPRAVATO® offers an innovative approach to treatment for patients experiencing a psychiatric emergency due to major depressive disorder (MDD-PE)
SPRAVATO® dosing schedule
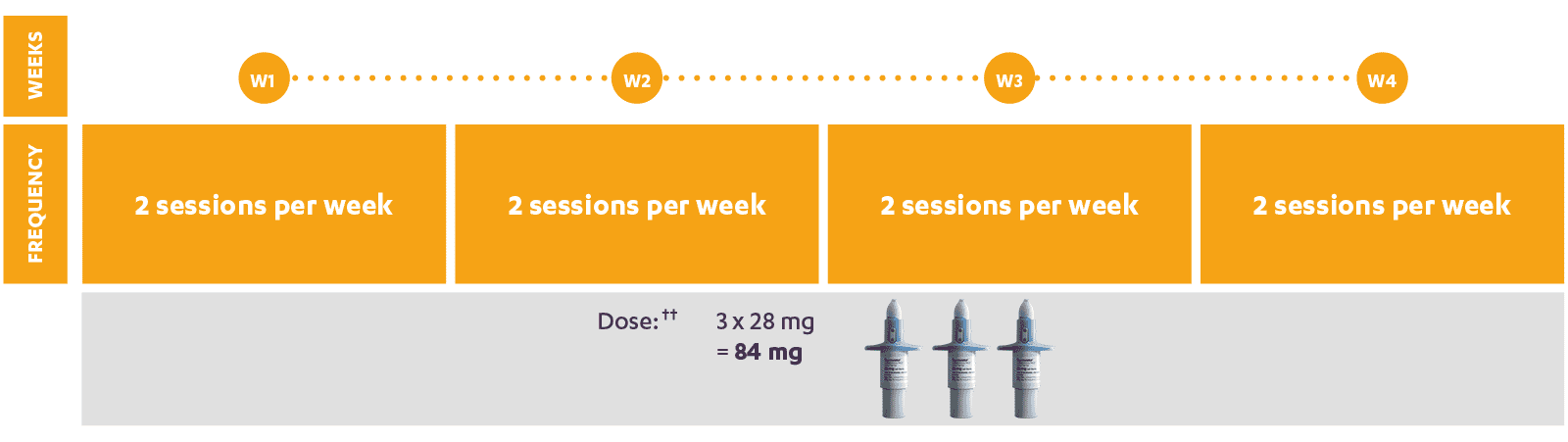
SPRAVATO®, co-administered with oral antidepressant therapy, is indicated in adults with a moderate to severe episode of Major Depressive Disorder, as acute short-term treatment, for the rapid reduction of depressive symptoms, which according to clinical judgement constitute a psychiatric emergency.‡‡
Dosage reduction from 84 mg to 56 mg should be made based on tolerability. SPRAVATO® must be taken alongside oral AD therapy, after the 4 weeks of treatment, this oral AD therapy should be continued, per clinical judgement. In these patients, treatment with SPRAVATO® should be a part of the comprehensive care plan.
SPRAVATO® does not require dose adjustment to account for:

Weight

Gender

Renal impairment§

Mild hepatic impairment||

Concomitant medications¶
Certain medications should be used with caution in combination with SPRAVATO®¶
Spravato, in combination with a SSRI or SNRI, is indicated for adults with treatment-resistant Major Depressive Disorder, who have not responded to at least two different treatments with antidepressants in the current moderate to severe depressive episode.
Spravato, co-administered with oral antidepressant therapy, is indicated in adults with a moderate to severe episode of Major Depressive Disorder, as acute short-term treatment, for the rapid reduction of depressive symptoms, which accordingly to clinical judgment constitute a psychiatric emergency. SPRAVATO® was investigated in adult patients with moderate to severe MDD who had affirmative responses to MINI questions B3 (“Think [even momentarily] about harming or of hurting or of injuring yourself: with at least some intent or awareness that you might die as a result; or think about suicide [i.e., about killing yourself]?”) and B10 (“Intend to act on thoughts of killing yourself in the past 24 hours?”). In these patients, treatment with SPRAVATO® should be part of the comprehensive clinical care plan. NOTE: The effectiveness of SPRAVATO® in preventing suicide or in reducing suicidal ideation or behaviour has not been demonstrated. Use of SPRAVATO® does not preclude the need for hospitalisation if clinically warranted, even if patients experience improvement after an initial dose of SPRAVATO®.
* Evidence of therapeutic benefit should be evaluated at the end of the induction phase to determine the need for continued therapy.
** Dosing frequency should be individualised to the lowest frequency to maintain remission/response.
† Efficacy of SPRAVATO® in Japanese patients has been studied, but not established.
‡ Among elderly patients, all dose changes should be in 28 mg increments.
§ There is no clinical experience with SPRAVATO® nasal spray in patients on renal dialysis.
|| No dosage adjustment is necessary in patients with mild (Child-Pugh class A) or moderate (Child-Pugh class B) hepatic impairment. SPRAVATO® has not been studied in patients with severe hepatic impairment (Child-Pugh class C). Use in this population is not recommended.
¶ A risk/benefit analysis and careful monitoring should be conducted when using SPRAVATO® with CNS depressants, psychostimulants, or monoamine oxidase inhibitors (MAOIs).
# Based on a post hoc analysis of patients in the tailored frequency phase of the SUSTAIN-2 trial, as assessed by CGI-S. Remission defined as MADRS ≤12. Frequency of administration was assessed and adjusted depending on whether remission was achieved. For patients in remission, treatment frequency was either maintained every 2 weeks or decreased from 1 session per week to every 2 weeks. For patients not in remission, treatment frequency was either maintained at 1 session per week or increased from every 2 weeks to 1 session per week until next assessment.
†† For adult patients (<65 years).
‡‡ The effectiveness of SPRAVATO® in preventing suicide or in reducing suicidal ideation or behaviour has not been demonstrated. Use of SPRAVATO® does not preclude the need for hospitalisation if clinically warranted, even if patients experience improvement after an initial dose of SPRAVATO®.
Abbreviations
AD, antidepressant; CGI-S, Clinical Global Impression Severity scale; CNS, central nervous system; MADRS, The Montgomery-Åsberg Depression Rating Scale; MAOI, monoamine oxidase inhibitor; MDD, major depressive disorder; MDD-PE, psychiatric emergency due to major depressive disorder; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TRD, treatment-resistant depression.
For Adverse Events or safety related issues, please approach group mailbox: PVNEMA@its.jnj.com
For Product Quality Complaints and Temperature Excursions, please approach group mailbox: QANEMA@its.jnj.com
For Medical Information, please approach group mailbox: Medical-info@its.jnj.com
For Prescribing Information: